Urinary outcomes are significantly affected by nerve sparing quality during radical prostatectomy
Urology. 2013 Dec;82(6):1348-53. doi: 10.1016/j.urology.2013.06.067. Epub 2013 Oct 3.
Kaye DR1, Hyndman ME, Segal RL, Mettee LZ, Trock BJ, Feng Z, Su LM, Bivalacqua TJ, Pavlovich CP.
Abstract
OBJECTIVE:
To assess the effect of nerve sparing (NS) quality on self-reported patient urinary outcomes after radical prostatectomy.
METHODS:
A total of 102 preoperatively potent men underwent laparoscopic or robotic radical prostatectomy; NS was prospectively graded at surgery using a 0-4 scale/neurovascular bundle. Urinary functional outcomes were measured by validated Expanded Prostate Cancer Index Composite questionnaire at baseline and follow-up time points (1, 3, 6, 9, and 12 months) in 99 men who underwent various degrees of NS. Mixed linear regression was used to analyze the effect of NS quality and other clinical factors on urinary outcomes.
RESULTS:
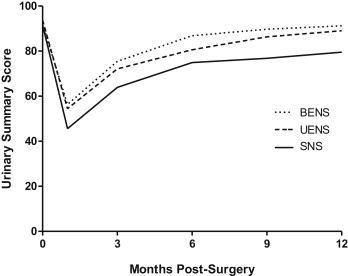
Patients with at least 1 neurovascular bundle spared completely, along with its supportive tissues (NS grade 4/4), noted significantly improved Expanded Prostate Cancer Index Composite urinary functional and continence outcomes as early as 1 month postoperatively and up to 12 months. Significantly less urinary bother was also noted in these men by 9-12 months postoperatively. Multivariate analysis revealed that bilateral or unilateral excellent NS (at least 1 bundle graded 4/4), increasing time from surgery, young patient age, and lower body mass index positively and significantly affected urinary functional outcomes, including pad use. Men who received excellent unilateral NS recovered urinary function about as well as men who had both neurovascular bundles spared in similar fashion.
CONCLUSION:
The quality of NS significantly influences patient-defined urinary functional convalescence. Completely sparing at least 1 neurovascular bundle along with its supportive tissues has a dramatic effect on the recovery of urinary continence and quality of life in preoperatively potent men.
