Alberta Prostate Cancer Registry & Biorepository
Biorepository
In many instances prostate cancer remains a mystery for researchers. At present, diagnostic tests lack accuracy and there is not a real prognostication tool to differentiate aggressive cancers vs indolent (less aggressive) ones. Thinking is changing in terms of treatment and today more than ever, the clinical and academic research communities need resources to measure outcomes associated with different treatment modalities through comprehensive registries and high quality biospecimens to test new biomarkers that can help better diagnose prostate cancer and predict disease outcomes by establishing biorepositories.
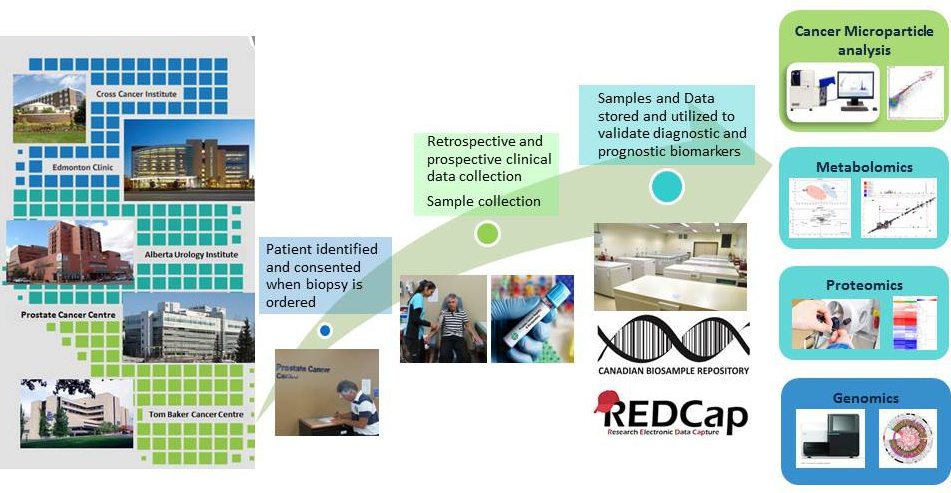
The Alberta Prostate Cancer BioRepository allows APCaRI team members application of state-of-the-art genomic, proteomic, metabolomic and transcriptomic analyses to the development of new and better tests for prostate cancer. Clinical validation of these tests requires large numbers of high quality patient biosamples linked to accurate and complete clinical outcome data.
We offer:
The Alberta Prostate Cancer Biorepository provides biofluid solutions for research. Academic and industry-based researchers worldwide can benefit from biofluids of high quality, diversity and integrity. Five academic teaching hospitals across Alberta currently collect samples and data from participating donors.
Access procedures for researchers are streamlined while ensuring high ethical standards and protection of donor privacy and confidentiality.
Samples Available from participants with prostate cancer and age-matched men with negative biopsy
- Serum (400uL/vial)
- Plasma (400uL/vial)
- Buffy Coat (~300uL/vial)
- Red Blood Cells (400uL/vial)
- Urine (400uL/vial)
- Semen (~400uL/vial)
Clinical Information
Clinical registries improve care by arming doctors and teams treating prostate cancer with information about how their outcomes compare with international standards and other locales. The Alberta Prostate Cancer Registry allows us to monitor the patterns of care and outcomes of men diagnosed with prostate cancer throughout Alberta, and provide a valuable tool to track how our translational research efforts are impacting outcomes over time. The registry is linked to a biorepository containing patient specimens that will drive translational research and enable personalized approaches to prostate cancer diagnosis and treatment.
- Demographic information and co-morbidities
- Family history of prostate cancer
- Pathology and diagnosis details
- Clinical and pathological staging
- Treatment history
- Outcomes
- Biospecimen collection, sample availability and processing details
The APCaRI Prostate Cancer Registry and Biorepository is a province-wide comprehensive resource that incorporates all prostate cancer patients in Alberta. Biobanking samples from Alberta prostate cancer patients collected through the journey of diagnosis and treatment will translate into enormous benefits because it will lead to tests that are directly applicable to Albertans.
By the end of 2019 Albertans and the health research community worldwide will have a comprehensive registry and biorepository containing personal, demographic, health and prostate cancer specific information along with biospecimens including blood (serum, plasma, buffy coat, and red blood cells), urine, semen and tissue from close to 9000 patients collected pre-diagnosis and at other multiple time points across the disease continuum.
In association with the Alberta Cancer Research Biorepository, our biorepository is utilizing cutting edge biobanking technology and supplies to guarantee that we have excellent quality samples managed with the highest standards and that we will have enough samples and data to support research for many years to come.
You can help us make an impact by donating biospecimens or by supporting our research.