Quantitative Analysis of human Cancer Cell Extravasation Using Intravital Imaging
Methods Mol Biol. 2016;1458:27-37
Willetts L, Bond D, Stoletov 1, Lewis JD
Abstract
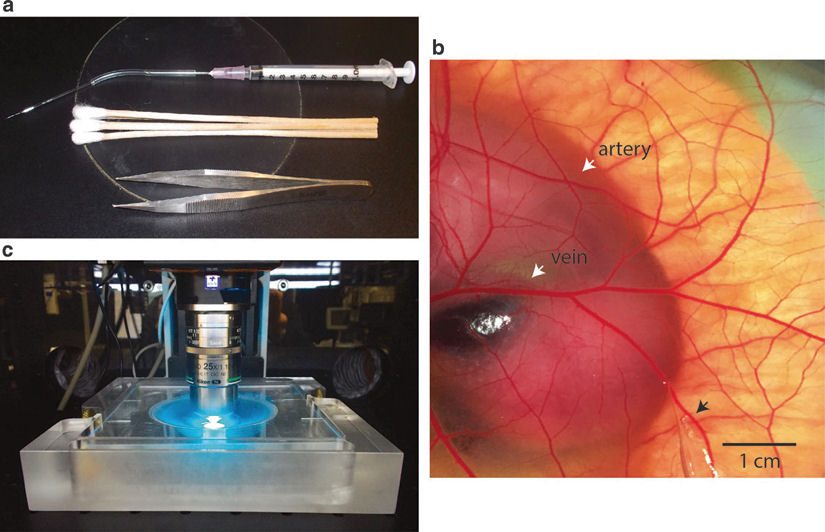
Metastasis, or the spread of cancer cells from a primary tumor to distant sites, is the leading cause of cancer-associated death. Metastasis is a complex multi-step process comprised of invasion, intravasation, survival in circulation, extravasation, and formation of metastatic colonies. Currently, in vitro assays are limited in their ability to investigate these intricate processes and do not faithfully reflect metastasis as it occurs in vivo. Traditional in vivo models of metastasis are limited by their ability to visualize the seemingly sporadic behavior of where and when cancer cells spread (Reymond et al., Nat Rev Cancer 13:858-870, 2013). The avian embryo model of metastasis is a powerful platform to study many of the critical steps in the metastatic cascade including the migration, extravasation, and invasion of human cancer cells in vivo (Sung et al., Nat Commun 6:7164, 2015; Leong et al., Cell Rep 8, 1558-1570, 2014; Kain et al., Dev Dyn 243:216-28, 2014; Leong et al., Nat Protoc 5:1406-17, 2010; Zijlstra et al., Cancer Cell 13:221-234, 2008; Palmer et al., J Vis Exp 51:2815, 2011). The chicken chorioallantoic membrane (CAM) is a readily accessible and well-vascularized tissue that surrounds the developing embryo. When the chicken embryo is grown in a shell-less, ex ovo environment, the nearly transparent CAM provides an ideal environment for high-resolution fluorescent microcopy approaches. In this model, the embryonic chicken vasculature and labeled cancer cells can be visualized simultaneously to investigate specific steps in the metastatic cascade including extravasation. When combined with the proper image analysis tools, the ex ovo chicken embryo model offers a cost-effective and high-throughput platform for the quantitative analysis of tumor cell metastasis in a physiologically relevant in vivo setting. Here we discuss detailed procedures to quantify cancer cell extravasation in the shell-less chicken embryo model with advanced fluorescence microscopy techniques.
