Ankyrin G expression is associated with androgen receptor stability, invasiveness, and lethal outcome in prostate cancer patients.
J Mol Med (Berl). 2016 Dec;94(12):1411-1422
Wang T, Abou-Ouf H, Hegazy SA, Alshalalfa M, Stoletov K, Lewis J, Donnelly B, Bismar TA
Abstract
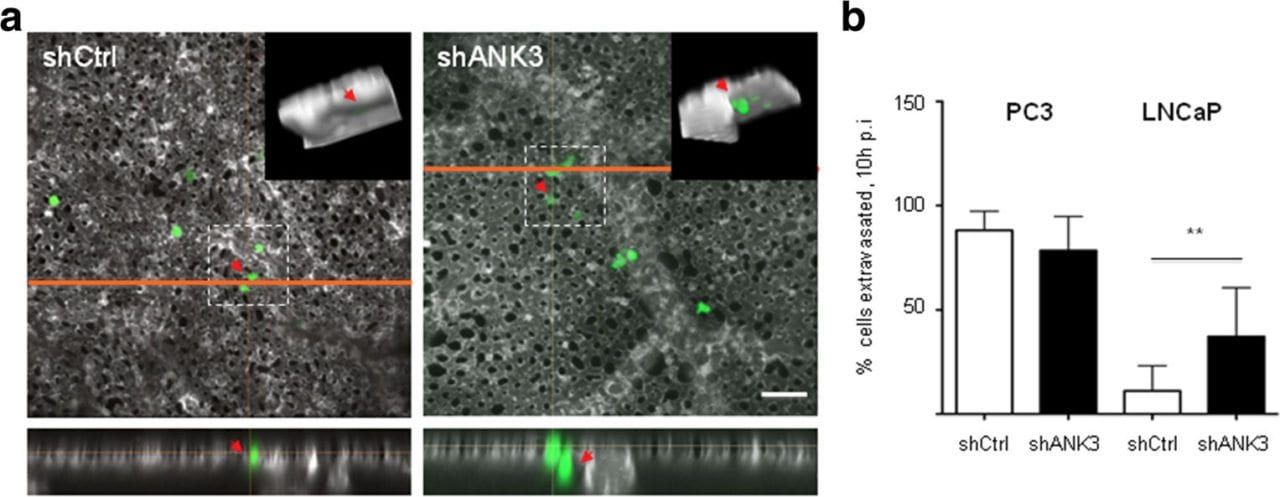
Ankyrin G (ANK3) is a member of the Ankyrin family, which functions to provide cellular stability by anchoring the cytoskeleton to the plasma membrane. Deregulation of ANK3 expression has been observed in multiple human cancers but its mechanism remains unknown. ANK3 expression in relation to disease progression and patients’ outcome was investigated in two cohorts of prostate cancer (PCA). Mechanistic studies were carried out in vitro and in vivo using several PCA cell lines and the avian embryo model. Silencing ANK3 resulted in significant reduction of cell proliferation through an AR-independent mechanism. Decreased ANK3 expression delayed S phase to G2/M cell cycle transition and reduced the expression of cyclins A and B. However, cells with knocked-down ANK3 exhibited significant increase in cell invasion through an AR-dependent mechanism. Furthermore, we found that ANK3 is a regulator of AR protein stability. ANK3 knockdown also promoted cancer cell invasion and extravasations in vivo using the avian embryo model (p < 0.01). In human samples, ANK3 expression was dramatically upregulated in high grade intraepithelial neoplasia (HGPIN) and localized PCA (p < 0.0001). However, it was downregulated castration resistant stage (p < 0.0001) and showed inverse relation to Gleason score (p < 0.0001). In addition, increased expression of ANK3 in cancer tissues was correlated with better cancer-specific survival of PCA patients (p = 0.012).
KEY MESSAGE:
Silencing ANK3 results in significant reduction of cell proliferation through an AR-independent mechanism. ANK3 knockdown results in significant increase in cell invasion through an AR-dependent mechanism. ANK3 is a regulator of AR protein stability. ANK3 knockdown also promotes cancer cell invasion and extravasation in vivo using the avian embryo model.
