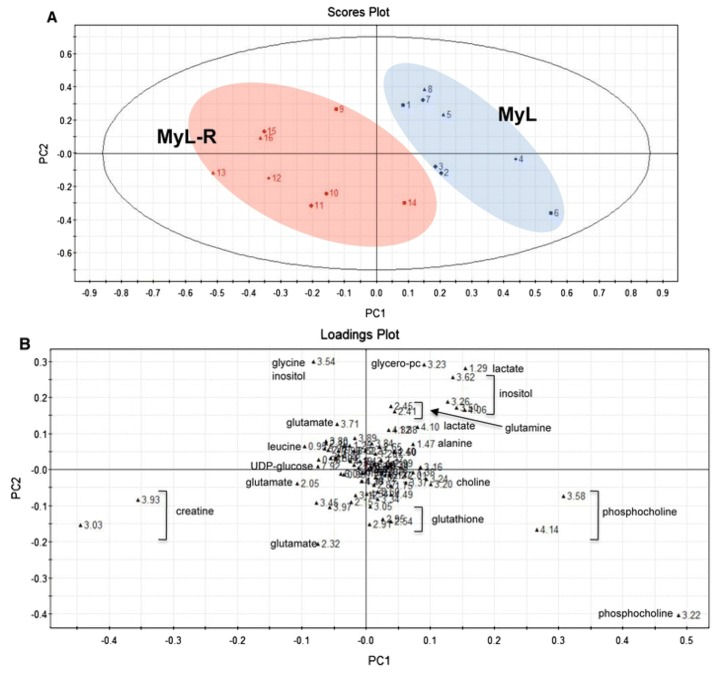
The Future of NMR Metabolomics in Cancer Therapy: Towards Personalizing Treatment and Developing Targeted Drugs?
Abstract
There has been a recent shift in how cancers are defined, where tumors are no longer simply classified by their tissue origin, but also by their molecular characteristics. Furthermore, personalized medicine has become a popular term and it could start to play an important role in future medical care. However, today, a “one size fits all” approach is still the most common form of cancer treatment. In this mini-review paper, we report on the role of nuclear magnetic resonance (NMR) metabolomics in drug development and in personalized medicine. NMR spectroscopy has successfully been used to evaluate current and potential therapies, both single-agents and combination therapies, to analyze toxicology, optimal dose, resistance, sensitivity, and biological mechanisms. It can also provide biological insight on tumor subtypes and their different responses to drugs, and indicate which patients are most likely to experience off-target effects and predict characteristics for treatment efficacy. Identifying pre-treatment metabolic profiles that correlate to these events could significantly improve how we view and treat tumors. We also briefly discuss several targeted cancer drugs that have been studied by metabolomics. We conclude that NMR technology provides a key platform in metabolomics that is well-positioned to play a crucial role in realizing the ultimate goal of better tailored cancer medicine.
