The Alberta Prostate Cancer Biorepository is certified by CTRNet
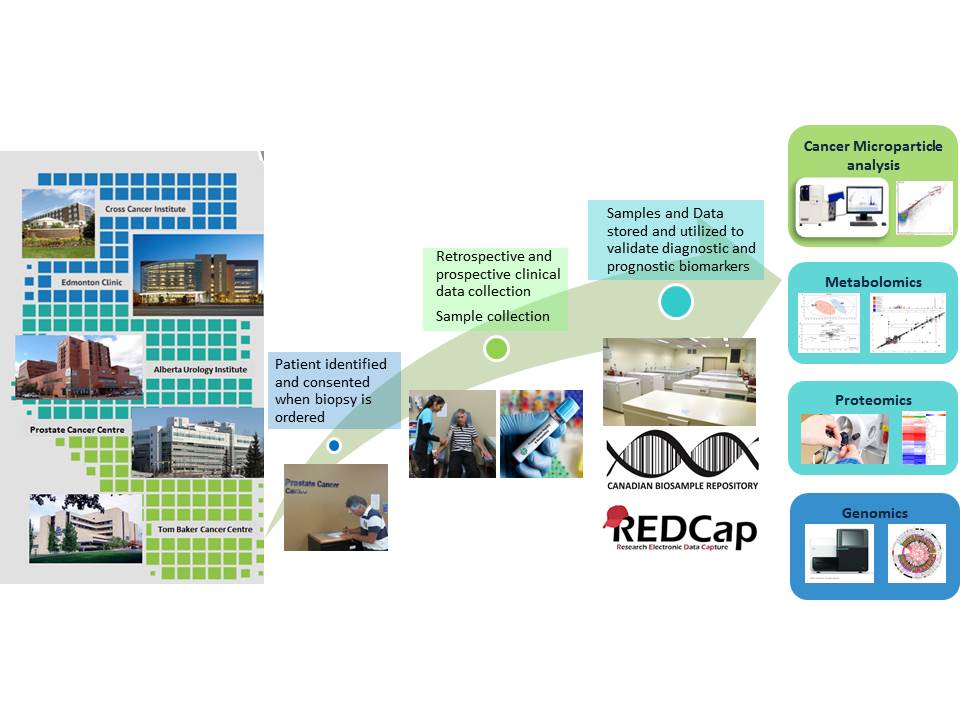
APCaRI is committed to collecting, processing and storing the samples you donate with the highest quality standards and as a result, after evaluation of our processes, the CTRNet (Canadian Tissue Repository Network) awarded us with their certification.
CTRNet is a translational cancer research resource, funded by Canadian Institutes of Health Research, that furthers Canadian health research by linking cancer researchers with provincial tumour banks.
The benefits of working with CTRNet include:
- The ability for researchers to search for quality controlled tissue samples from Canada’s leading tumour banks in one central location and for biobanks to display and make their biospecimens available for research users.
- Learning opportunities in tissue handling, research design and relevant technology training and innovations.
- Invitation to CTRNet workshops and conferences.