Our First Participant!
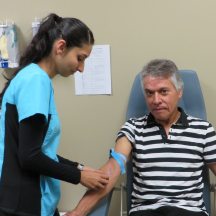
Thanks to the participation from men with suspected prostate cancer and men diagnosed with prostate cancer, we will be able to measure if our “tests” can reveal the true nature of prostate cancer and if the tests or biomarkers can diagnose prostate cancer and tell us what cancers are more aggressive.
As part of the Alberta Prostate Registry and Biorepository, patients will be entered into our study, in which blood and other samples are collected over time and their health outcomes are recorded over many years. Patients will follow standard medical advice and care through their doctors. Our team collect biospecimens and information related to general health and cancer behavior over time.
Rather than being frightened by the word ‘cancer’, we want to learn how to predict serious and morbid prostate cancer complications well before they happen, so that we can weigh carefully the pros and cons of available treatments.
In the process, we expect to identify new and important advantage points for better therapies to be developed. The word “cancer” may be scary, but what is truly scary is unawareness.
“It makes me very happy to be able to contribute to find better ways to diagnose prostate cancer.”