Real-time visualization and quantitation of vascular permeability in vivo: implications for drug delivery.
Abstract
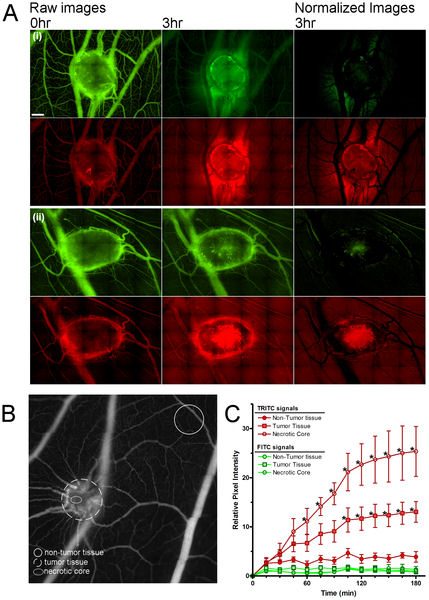
The leaky, heterogeneous vasculature of human tumors prevents the even distribution of systemic drugs within cancer tissues. However, techniques for studying vascular delivery systems in vivo often require complex mammalian models and time-consuming, surgical protocols. The developing chicken embryo is a well-established model for human cancer that is easily accessible for tumor imaging. To assess this model for the in vivo analysis of tumor permeability, human tumors were grown on the chorioallantoic membrane (CAM), a thin vascular membrane which overlays the growing chick embryo. The real-time movement of small fluorescent dextrans through the tumor vasculature and surrounding tissues were used to measure vascular leak within tumor xenografts. Dextran extravasation within tumor sites was selectively enhanced an interleukin-2 (IL-2) peptide fragment or vascular endothelial growth factor (VEGF). VEGF treatment increased vascular leak in the tumor core relative to surrounding normal tissue and increased doxorubicin uptake in human tumor xenografts. This new system easily visualizes vascular permeability changes in vivo and suggests that vascular permeability may be manipulated to improve chemotherapeutic targeting to tumors.
